Research
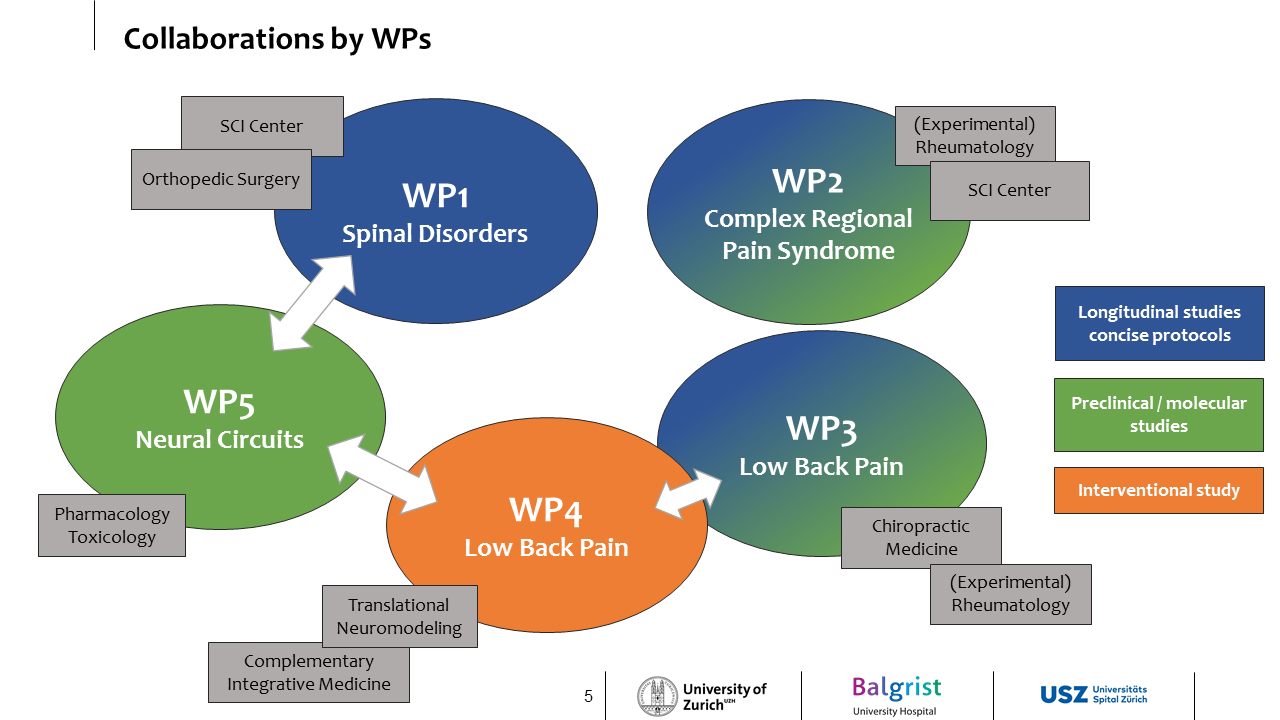
WP1 – Pain Phenotyping in spinal disorders (PIs: A. Curt / M. Hubli / M. Farshad)
WP2 – Complex regional pain syndrome working group (PIs: O. Distler / S. Dudli / M. Hubli / F. Brunner)
WP3 – Low back pain working group (PIs: P. Schweinhardt / S. Dudli / O. Distler)
WP4 – Cognitive intervention in low back pain (PIs: K.E. Stephan / C. Witt)
WP5 - Neural circuits of pain (PI: H.U. Zeilhofer)
PAIN - A COMPLEX PATHOPHYSIOLOGY
Chronic pain continues to pose a major, unmet challenge for modern medicine with tremendous global healthcare costs affecting ~20% of the population. Persistent pain is multi-factorial and likely to be maintained in many instances through pathophysiological changes along the neuraxis. The CRPP Pain fosters a
multi-disciplinary approach (neurology, rheumatology, orthopedics, chiropractic medicine, neurobiology and neuromodeling) to better understand the relative contributions of different pathophysiological processes in multiple neural systems, i.e., peripheral nerves, spinal cord, brainstem and cortex, while also incorporating
cognitive and affective processes.
FROM PHENOTYPES TO MECHANISMS
In order to deconstruct the complex pain phenotype and obtain pointers to common potential underlying mechanisms, the CRPP Pain employed cross-sectional studies across different chronic pain conditions, i.e., neuropathic pain following spinal cord injury (SCI), complex regional pain syndrome (CRPS) and low back pain (LBP). For the renewal application, the gained knowledge is taken one step further, informing longitudinal study designs targeting the progression/prediction of clinical pain in identified distinct phenotypes.
Solid prospective longitudinal studies are needed to understand the evolution and progression of pathophysiological processes that occur from the transition from an acute to a chronic stage and alongside fluctuations of clinical manifestation of pain, e.g., pain episodes/relapses.
WP1 will determine the most predictive measures for the development and severity of neuropathic pain in spinal disorders. In particular, changes in the pain phenotype in degenerative cervical myelopathy (DCM) will be monitored by a well-established test battery including clinical pain characterization, sensory profiles, experimental pain paradigms and neurophysiology. Comparable test batteries will also be applied in a longitudinal fashion for CRPS (WP2) and LBP patients (WP3).
The two newly established working groups "CRPS" and "LBP" will employ a holistic investigation of biopsychophysical dimensions of pain.
WP2 will longitudinally characterize CRPS patients by molecular markers (skin and blood) in combination with pain phenotyping and psychological assessment.
The identification of key cells and their molecular changes in skin and blood can be used as biomarker for diagnosis or monitoring of CRPS and subgroups of patients. WP3 will link pain phenotypes in LBP patient outcomes and identify blood biomarkers that correlate with these pain phenotypes.
To this end, WP3 will investigate biological, psychophysical, psychological, and clinical alterations in chronic LBP patients andinvestigate which of them are likely to be pathophysiologically relevant. While WP3 mainly focuses on longitudinal biopsychophysical alterations in LBP, WP4 will examine the effectiveness of two novel digitally delivered interventions, compared to usual care, in LBP patients.
Specifically, WP4 will conduct a randomized controlled trial that tests whether in patients with subacute/chronic LBP receiving infiltration therapy, mindfulness-based and expectation-focused interventions - delivered as brief guided digital interventions – reduce pain.
Finally, WP5 on neural circuits for pain prediction and cold-induced analgesia enables a thorough basic, mechanistic investigation of several concepts applied in the above-mentioned WPs, such as endogenous pain modulation, analgesia by cold stimuli and pain expectation. WP5 will investigate whether descending corticospinal layer 5 pyramidal neurons serve physiological functions in endogenous pain control and delineate central circuits for cold-induced analgesia. Both projects will address phenomena (cold-induced analgesia and pain prediction) that we study in patients and healthy human volunteers (WP1 and 4, respectively).
PRECISION MEDICINE AS A COMMON VISION
Our common goal with this CRPP is to move from the current empirical therapeutic approaches, often with many attempts and yet dissatisfactory outcomes, to personalized targeting of disorder-specific mechanisms in chronic pain patients. We are convinced that information exchange between clinical disciplines, i.e. neurology,
rheumatology, chiropractic medicine, orthopedics, and basic research in neurobiology and neuromodeling, will provide a successful venue for the generation of novel knowledge regarding pain mechanisms underlying different diseases. This will afford a sound scientific basis for improved patient treatment and informed clinical trials.
Our truly inter-disciplinary approach is reflected in the newly established and well-attended courses of the CRPP Pain for undergraduate students (block course (Modul BME 355) at the Science Faculty, UZH) and ZNZ symposia ETH/UZH. National and international public outreach at the level of consumers (BrainFair, Scientifica) and
scientists (international conferences and workshops) are considered fundamental to increasing awareness of the achievements in pain research, and to foster research cooperation in a dynamic and highly specialized field.